Neon-22 contains 12 neutrons. which statement is true. – Neon-22 contains 12 neutrons. Which statement is true? Embark on a scientific exploration to uncover the mysteries surrounding this element’s composition and properties.
Neon, an inert gas with a myriad of applications, exists in various isotopic forms. Among them, neon-22 stands out with its unique characteristics, which stem from its specific neutron count.
Neon-22 and Its Composition
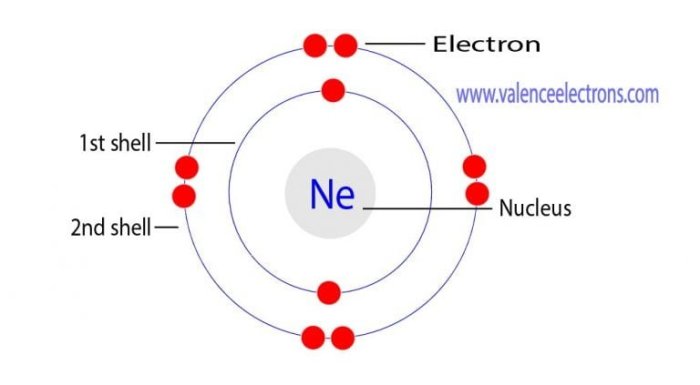
Neon-22 is an isotope of neon, an element with the atomic number 10. It has 10 protons and 10 electrons, giving it a neutral electrical charge. Isotopes are variations of the same element that differ in the number of neutrons in their atomic nuclei.
Neon-22 has 12 neutrons, giving it a total atomic mass of 22.
| Property | Neon-22 |
|---|---|
| Atomic number | 10 |
| Number of protons | 10 |
| Number of neutrons | 12 |
| Number of electrons | 10 |
| Atomic mass | 22 |
Neutron Count in Neon-22: Neon-22 Contains 12 Neutrons. Which Statement Is True.
The number of neutrons in an atom affects its atomic mass. Neon-22 has 12 neutrons, which contribute to its atomic mass of 22. Other isotopes of neon have different numbers of neutrons, resulting in different atomic masses.
- Neon-20: 10 neutrons
- Neon-21: 11 neutrons
- Neon-23: 13 neutrons
Properties of Neon-22
Neon-22 is a colorless, odorless, and non-flammable gas. It has a low reactivity and high thermal conductivity. The number of neutrons in neon-22 does not significantly affect its chemical properties, but it does influence its atomic mass and physical properties.
| Property | Neon-22 | Other Neon Isotopes |
|---|---|---|
| Reactivity | Low | Low |
| Thermal conductivity | High | High |
| Atomic mass | 22 | 20-23 |
Applications of Neon-22
Neon-22 has various practical applications due to its unique properties. It is commonly used in:
- Lighting: Neon-22 is used in fluorescent and neon lights, producing a bright red-orange glow.
- Medical imaging: Neon-22 is used as a tracer in positron emission tomography (PET) scans, helping diagnose and monitor various medical conditions.
- Scientific research: Neon-22 is used in particle accelerators and other scientific experiments.
Safety Considerations for Neon-22
Neon-22 is an inert gas, posing no chemical hazards. However, it can displace oxygen in enclosed spaces, creating an asphyxiation risk. When handling or using neon-22, proper safety measures should be taken:
- Ensure adequate ventilation in areas where neon-22 is present.
- Store and transport neon-22 in sealed containers.
- Dispose of neon-22 responsibly, following local regulations.
Top FAQs
Is neon-22 radioactive?
No, neon-22 is a stable isotope and does not exhibit radioactivity.
What is the atomic mass of neon-22?
Approximately 22 atomic mass units, due to its 10 protons and 12 neutrons.
How is neon-22 used in lighting?
Neon-22 is commonly employed in fluorescent lighting, producing a characteristic reddish-orange glow.